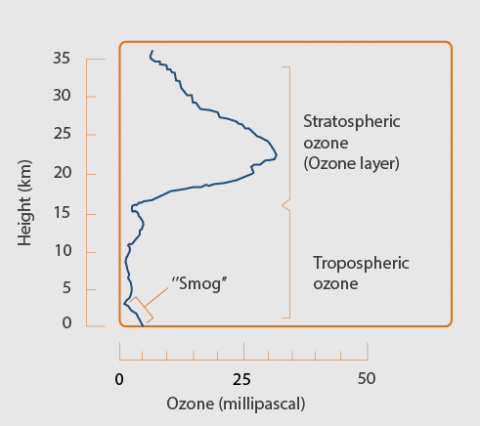
Ozone (O3), a molecule comprised of three oxygen atoms (O), is mainly found in two layers of our atmosphere:
- The stratosphere, a layer in the atmosphere between approximately 10 and 50 kilometers above the Earth’s surface, contains around 90% of the total atmospheric ozone amount. This stratospheric ozone is generally known as the “ozone layer”.
- The ozone in the troposphere, the lowest layer in the atmosphere that reaches from the Earth's surface to an altitude between 8 to 15 kilometres, accounts for the remaining 10% of ozone.
Ozone molecules are chemically the same in both layers, consisting of three oxygen atoms and sharing the same chemical formula O3. Nevertheless, they influence humans and other living creatures in a totally different way.
Ozone in the stratosphere absorbs dangerous UV radiation
Stratospheric ozone plays a critical role for the biosphere because it largely absorbs the damaging ultraviolet radiation, allowing only a small part of the radiation to reach the Earth.
Absorbing ultraviolet radiation, ozone is a source of heath and is in this way actually responsible for the formation of the stratosphere itself (which is a layer where temperature rises with increasing height). Ozone is essential to explain the temperature structure of the terrestrial atmosphere.
If unhindered by the filtering effect of the ozone, the full UV-B radiation would reach the Earth’s surface. As suggested by many experiments on plants and animals and clinical research on humans, an increased exposure to UV-B radiation has its damaging effects.
Ozone in the troposphere is poisonous substance
Tropospheric ozone, on the contrary, is a so-called secondary pollutant, a photo oxidant which is formed by the interaction of solar radiation with primary polluting precursors.
Tropospheric ozone is a poisonous substance that harms humans, animals and plants. It results from complex chemical reactions, with a substantial role for the following players:
- nitrogen oxides
- volatile organic compounds
- carbon monoxide
- temperature
- sunlight